Tumour Lysis Syndrome (TLS) is a life-threatening oncological emergency resulting from the rapid breakdown of malignant cells, leading to severe metabolic disturbances. It is most commonly associated with haematological malignancies, particularly high-grade lymphomas (e.g., Burkitt lymphoma) and acute leukaemias, but can also occur in solid tumours with high tumour burden.
Pathophysiology
TLS occurs due to the rapid release of intracellular contents into the bloodstream following cell lysis, either spontaneously or after initiating cytotoxic therapy. This results in:
- Hyperkalaemia – Due to the release of intracellular potassium.
- Hyperphosphataemia – From cellular phosphate release, leading to secondary hypocalcaemia.
- Hypocalcaemia – Due to calcium-phosphate precipitation.
- Hyperuricaemia – From nucleic acid breakdown and conversion to uric acid.
- Acute kidney injury (AKI) – Due to uric acid and calcium phosphate crystal deposition in the renal tubules.
Risk Factors
- High tumour burden (e.g., bulky lymphomas, acute leukaemias with high WBC count).
- Rapidly proliferating malignancies.
- Pre-existing renal impairment.
- Initiation of chemotherapy, corticosteroids, or targeted therapies. Venetoclax is a common precipitant.
Clinical Features
- Electrolyte disturbances: Muscle weakness, tetany, cardiac arrhythmias.
- Renal dysfunction: Oliguria, rising creatinine.
- Neurological symptoms: Seizures, confusion.
- Cardiac complications: Arrhythmias, sudden cardiac arrest.
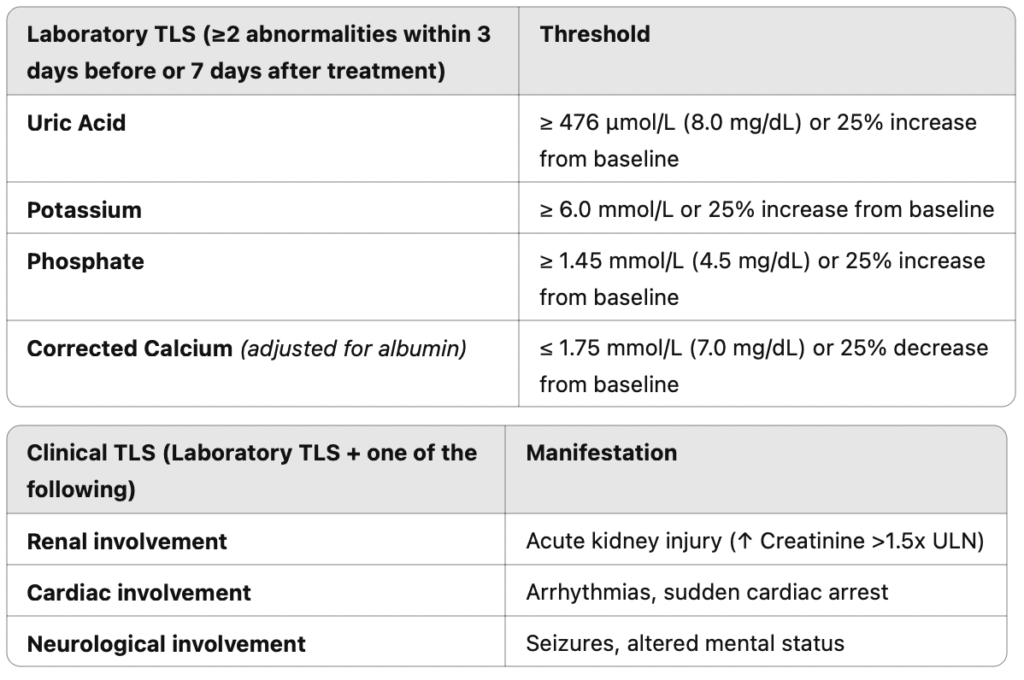
Diagnosis
TLS is diagnosed using Cairo-Bishop criteria, which classify cases into laboratory and clinical TLS based on metabolic abnormalities and organ dysfunction.
Prevention and Management
Prevention
- Risk stratification – Identifying high-risk patients before initiating treatment.
- Hydration – Aggressive IV fluids (e.g., 3L/m²/day) to promote renal clearance of metabolites.
- Allopurinol – Xanthine oxidase inhibitor used for prophylaxis in intermediate-risk patients.
- Rasburicase – Recombinant urate oxidase enzyme used in high-risk patients to rapidly degrade uric acid.
Rasburicase: Flat vs Weight-Based Dosing
Rasburicase dosing has traditionally been weight-based (0.2 mg/kg), but flat dosing (e.g., 3–7.5 mg as a single dose) is now increasingly used due to cost-effectiveness and similar efficacy. Studies suggest that a single fixed dose (e.g., 6 mg)can be sufficient in most cases, reducing drug expenditure while maintaining effective uric acid control.
Treatment of Established TLS
- IV fluids to enhance renal clearance.
- Rasburicase for hyperuricaemia.
- Electrolyte correction: Calcium gluconate for severe hypocalcaemia, potassium/phosphate binders as needed.
- Dialysis in refractory cases with severe AKI.
Conclusion
TLS is a potentially fatal condition requiring early recognition and aggressive management. Flat dosing of rasburicase is a cost-effective alternative to weight-based dosing with comparable efficacy. Junior doctors should be vigilant in identifying at-risk patients and initiating preventive strategies to improve outcomes.
Want to learn more? Visit HaematologyForDoctors.com!
Leave a Reply